Formula of a Hydrate
Safety:
Crucibles are VERY HOT; always handle them with tongs. DO NOT put hot crucibles on a balance! They cost $9.00 if you
break it. Crucibles are VERY FRAGILE. Never carry them around without a heat-proof pad under it. Wear goggles at all
times.
Introduction:
The water can easily be removed from a hydrate just by heating strongly. You will be weighing a hydrate and heating it to
remove the water (now called "anhydrous salt") and weigh it again. You can now find the percent of the anhydrous salt and the
water. By finding a mol ratio, you can find out how many moles of water there are per mol of anhydrous salt. This number goes
just before the H2O in the formula.
Sample Calculation-
An empty crucible has a mass of 12.770 grams. The crucible and hydrate have a mass of 13.454 grams. After heating, the
crucible and anhydrous salt have a mass of 13.010 grams. What is the formula of this hydrate of
MgSO4 . ?H2O?
Mass of hydrate = 13.454 - 12.770 = .684 grams
Mass of anhydrous salt = 13.010 - 12.770 = .240 grams
Mass of water = 13.454 - 13.010 = .444 grams
Moles of anhydrous salt = .240 grams MgSO4 x 1 mol MgSO4 = .00199 moles MgSO4
1 120.367 g MgSO4
Moles of water = .444 grams H2O x 1 mol H2O = .0246 moles H2O
1 18.0148 g H2O
Ratio of moles of water to moles of anhydrous salt = .0246/.00199 = 12
Therefore the formula is MgSO4 . 12H2O
Empirical and Molecular Formula
The empirical formula of a chemical compound is the simplest whole number ratio of atoms of each element present in a compound.
*NOTE*: All ionic compounds are empirical formulas.
Ex: C4H10 (molecular formula of butane) can be reduced to C2H5 (empirical formula)
Ex: What is the empirical formula of a compound containing 11.1% hydrogen and 88.9% oxygen by mass?
Assume you have 100 g.
The molecular formula is a multiple of the empirical formula that contains the actual number of atoms that combine to form a molecule.
*To calculate the multiple: n = molar mass of the compound
molar mass of the empirical formula
Ex: A molecule has an empirical formula of C2H5 and a molar mass of 58 g/mol. What is the molecular formula?
~ The metal comes first and then the non-metals after are put in alphabetical order~
*NOTE*: All ionic compounds are empirical formulas.
Ex: C4H10 (molecular formula of butane) can be reduced to C2H5 (empirical formula)
Ex: What is the empirical formula of a compound containing 11.1% hydrogen and 88.9% oxygen by mass?
Assume you have 100 g.
H: 11.1% x 1 mole/ 1g = 11.1 mol ---> 1
O: 88.9% x 1mole/ 16g = 55.6 mol --->5
Empirical formula= HO5Now to write the molecular formula using the empirical formula!
The molecular formula is a multiple of the empirical formula that contains the actual number of atoms that combine to form a molecule.
*To calculate the multiple: n = molar mass of the compound
molar mass of the empirical formula
Ex: A molecule has an empirical formula of C2H5 and a molar mass of 58 g/mol. What is the molecular formula?
MM C2H5 = 29 g/mol
n = 58g/mol / 29g/mol =2
2 x C2H5 = C4H10
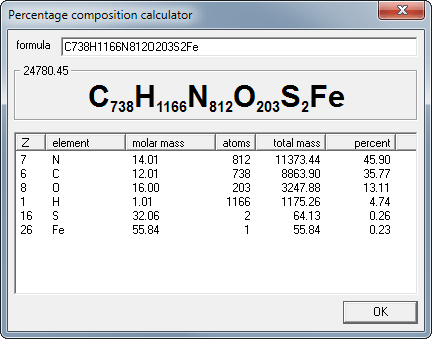
Percentage Composition
What is percentage composition?
The percent composition (percentage composition) of a compound is a relative measure of the mass of each different element present in the compound.
To calculate the percent composition (percentage composition) of a compound
Calculate the molecular mass (molecular weight, formula mass, formula weight), MM, of the compound
Calculate the total mass of each element present in the formula of the compound
Calculate the percent compositon (percentage composition): % by weight (mass) of element
= (total mass of element present ÷ molecular mass) x 100
Example 1.
What percentage of the mass of carbon dioxide (CO2) is made up by the carbon?
Solution:
first find the mass of the total compound.
C = 12.0 u x 1 atom = 12.0 u
O = 16.0 u x 2 atoms = 32.0 u
--------
44.0 u
next use the formula:
partial mass from carbon
% of the mass of CO2 that is made up by carbon = -------------------------- x 100
total mass of the CO2
12.0 u
% of the mass of CO2 that is made up by carbon = ------------------------- x 100
44.0 u
% of the mass of CO2 that is made up by carbon = 27.3%
Answer = 27.3%
Now, it is more typical to be asked what the percentage composition of the entire compound is. In the example above, you can assume that if carbon makes up approximately 27% of the mass of carbon dioxide then oxygen makes up about 73%, for the total must be 100%.
Example 2.
What is the percentage composition of glucose (C6H12O6) ?
solution:
find the mass of the entire molecule,
C = 12.0 u x 6 atoms = 72.0 u
H = 1.01 u x 12 atoms = 12.1 u
O = 16.0 u x 6 atoms = 96.0 u
----------
180 u
Then use the formula for each element in the compound:
partial mass from element
% of the mass of the compound that is made up by an element = -------------------------- x 100
total mass of the compound
72.0 u
% for Carbon = ---------------- x 100 = 40.0%
180 u
12.1 u
% for Hydrogen = ---------------- x 100 = 6.7 %
180 u
96.0 u
% for Oxygen = --------------- x 100 = 53.3%
180 u
One way to check your answer is to make sure that all of the percentages add up to approximately 100%. (i.e. 40.0% + 6.7% + 53.3% = 100%) Your total may be off by a few tenths of a percent, due to rounding.
Example 3.
Calculate the percent by weight of each element present in ammonium phosphate [(NH4)3PO4]
Calculate the molecular mass (MM) of (NH4)3PO4:
MM = 3x[14.01 + (4 x 1.008)] + 30.97 + (4 x 16.00) = 3 x [14.01 + 4.032] + 30.97 + 64.00 = (3 x 18.042) + 30.97 + 64.00 = 54.126 + 30.97 + 64.00 = 149.096
Calculate the total mass of N present:
3 N are present, mass = 3 x 14.01 = 42.03
Calculate the percent by mass of N present in (NH4)3PO4:
%N = (mass N ÷ MM) x 100 = (42.03 ÷ 149.096) x 100 = 28.19%
Calculate the total mass of H present:
12 H are present in the formula, mass = 12 x 1.008 = 12.096
Calculate the percent by mass of H present in (NH4)3PO4:
%H = (mass H ÷ MM) x 100 = (12.096 ÷ 149.096) x 100 = 8.11%
Calculate the total mass of P present:
1 P is present in the formula, mass = 30.97
Calculate the percent by mass P in (NH4)3PO4:
%P = (mass P ÷ MM) x 100 = (30.97 ÷ 149.096) x 100 = 20.77%
Calculate the total mass of O present:
4 O are present in the formula, mass = 4 x 16.00 = 64.00
Calculate the percent by mass of O in (NH4)3PO4:
%O = (mass O ÷ MM) x 100 = (64.00 ÷ 149.096) x 100 = 42.93%
The answers above are probably correct if %N + %H + %P + %O =100, that is,
28.19 + 8.11 + 20.77 + 42.93 = 100 %
Here is a video on YouTuBe:
The percent composition (percentage composition) of a compound is a relative measure of the mass of each different element present in the compound.
To calculate the percent composition (percentage composition) of a compound
Calculate the molecular mass (molecular weight, formula mass, formula weight), MM, of the compound
Calculate the total mass of each element present in the formula of the compound
Calculate the percent compositon (percentage composition): % by weight (mass) of element
= (total mass of element present ÷ molecular mass) x 100
Example 1.
What percentage of the mass of carbon dioxide (CO2) is made up by the carbon?
Solution:
first find the mass of the total compound.
C = 12.0 u x 1 atom = 12.0 u
O = 16.0 u x 2 atoms = 32.0 u
--------
44.0 u
next use the formula:
partial mass from carbon
% of the mass of CO2 that is made up by carbon = -------------------------- x 100
total mass of the CO2
12.0 u
% of the mass of CO2 that is made up by carbon = ------------------------- x 100
44.0 u
% of the mass of CO2 that is made up by carbon = 27.3%
Answer = 27.3%
Now, it is more typical to be asked what the percentage composition of the entire compound is. In the example above, you can assume that if carbon makes up approximately 27% of the mass of carbon dioxide then oxygen makes up about 73%, for the total must be 100%.
Example 2.
What is the percentage composition of glucose (C6H12O6) ?
solution:
find the mass of the entire molecule,
C = 12.0 u x 6 atoms = 72.0 u
H = 1.01 u x 12 atoms = 12.1 u
O = 16.0 u x 6 atoms = 96.0 u
----------
180 u
Then use the formula for each element in the compound:
partial mass from element
% of the mass of the compound that is made up by an element = -------------------------- x 100
total mass of the compound
72.0 u
% for Carbon = ---------------- x 100 = 40.0%
180 u
12.1 u
% for Hydrogen = ---------------- x 100 = 6.7 %
180 u
96.0 u
% for Oxygen = --------------- x 100 = 53.3%
180 u
One way to check your answer is to make sure that all of the percentages add up to approximately 100%. (i.e. 40.0% + 6.7% + 53.3% = 100%) Your total may be off by a few tenths of a percent, due to rounding.
Example 3.
Calculate the percent by weight of each element present in ammonium phosphate [(NH4)3PO4]
Calculate the molecular mass (MM) of (NH4)3PO4:
MM = 3x[14.01 + (4 x 1.008)] + 30.97 + (4 x 16.00) = 3 x [14.01 + 4.032] + 30.97 + 64.00 = (3 x 18.042) + 30.97 + 64.00 = 54.126 + 30.97 + 64.00 = 149.096
Calculate the total mass of N present:
3 N are present, mass = 3 x 14.01 = 42.03
Calculate the percent by mass of N present in (NH4)3PO4:
%N = (mass N ÷ MM) x 100 = (42.03 ÷ 149.096) x 100 = 28.19%
Calculate the total mass of H present:
12 H are present in the formula, mass = 12 x 1.008 = 12.096
Calculate the percent by mass of H present in (NH4)3PO4:
%H = (mass H ÷ MM) x 100 = (12.096 ÷ 149.096) x 100 = 8.11%
Calculate the total mass of P present:
1 P is present in the formula, mass = 30.97
Calculate the percent by mass P in (NH4)3PO4:
%P = (mass P ÷ MM) x 100 = (30.97 ÷ 149.096) x 100 = 20.77%
Calculate the total mass of O present:
4 O are present in the formula, mass = 4 x 16.00 = 64.00
Calculate the percent by mass of O in (NH4)3PO4:
%O = (mass O ÷ MM) x 100 = (64.00 ÷ 149.096) x 100 = 42.93%
The answers above are probably correct if %N + %H + %P + %O =100, that is,
28.19 + 8.11 + 20.77 + 42.93 = 100 %
Here is a video on YouTuBe:
Mole Map
Grams ↔ Moles
Number of moles = (# of grams) ÷ (molar mass)
Number of grams = (# of moles) × (molar mass)
Example 1:How many moles are in 5 grams of O2?
The molar mass of O2 = 16.00 g/mole x 2 (for 2 atoms of oxygen) or 32.00 g/mole.
5 grams of O2 ÷ (32 g/mole) = 0.15625 moles
Example 2:
How many grams does 4 moles of NH3 weigh?
The molar mass of NH3 = 14.01 + (3 × 1.01) = 17.04 g/mole
4 moles × 17.04 g/mole = 68.16 grams
Number of grams = (# of moles) × (molar mass)
Example 1:How many moles are in 5 grams of O2?
The molar mass of O2 = 16.00 g/mole x 2 (for 2 atoms of oxygen) or 32.00 g/mole.
5 grams of O2 ÷ (32 g/mole) = 0.15625 moles
Example 2:
How many grams does 4 moles of NH3 weigh?
The molar mass of NH3 = 14.01 + (3 × 1.01) = 17.04 g/mole
4 moles × 17.04 g/mole = 68.16 grams
Atoms ↔ Moles
Number of moles = (# of atoms) ÷ (6.02x10^23)
Number of atoms = (# of moles) × (6.02x10^23)
Number of atoms = (# of moles) × (6.02x10^23)
Example 1:
1.65 × 1024 atoms of Magnesium?
1.65 × 1024 atoms ÷ (6.02 × 1023) = 2.74 moles of Mg
Mole Conversions
Above are 2 videos that show how grams are converted to moles and how moles are converted to grams.
The atomic mass is the mass of the mass of one atom of an element in ATOMIC MASS UNITS (u). This number can be found on the periodic table.
Example:
The atomic mass of Magnesium is 24.3 u.
The formula mass is the total mass of all atoms in a covalent, organic, or polyatomic compound in ATOMIC MASS UNITS (u).
Example:
The formulas mass of NaCl is: 23.0 + 35.5 = 58.5 u.
The molecular mass is the total mass of all atoms in an ionic compound in ATOMIC MASS UNITS (u).
Example:
The molecular mass of CO is: 12.0 + 16.0 = 28.0 u.
We now know how to do conversions:
(a) from particles/atoms/formula units/molecules ----> moles
(b) from moles ----> particles/atoms/formula units/molecules
(c) from grams ----> moles
and (d) from moles ----> grams
*With all these conversions, the molar mass is needed.
The molar mass is the same number of the molecular, atomic, or formula masses, except expressed in grams per mole (g/mole).

The atomic mass is the mass of the mass of one atom of an element in ATOMIC MASS UNITS (u). This number can be found on the periodic table.
Example:
The atomic mass of Magnesium is 24.3 u.
The formula mass is the total mass of all atoms in a covalent, organic, or polyatomic compound in ATOMIC MASS UNITS (u).
Example:
The formulas mass of NaCl is: 23.0 + 35.5 = 58.5 u.
Example:
The molecular mass of CO is: 12.0 + 16.0 = 28.0 u.
We now know how to do conversions:
(a) from particles/atoms/formula units/molecules ----> moles
(b) from moles ----> particles/atoms/formula units/molecules
(c) from grams ----> moles
and (d) from moles ----> grams
*With all these conversions, the molar mass is needed.
The molar mass is the same number of the molecular, atomic, or formula masses, except expressed in grams per mole (g/mole).

Mole day is at 6:22 on October 23 (10/23)
Equal Numbers in Equal Volumes: Avogadro
Relative Mass:
Expressed by company it mathematically to the mass of another object.
Avogardo's Law:
Equal volume of all gases under same conditions of temperature and pressure contain equal no of molecules.
Things to understand about Avogadro's number:
• It is a number, just as is "dozen", and thus is dimensionless; you can think of Avogadro's number as the "chemist's dozen".
• It is a huge number, far greater in magnitude than we can visualize;
• Its practical use is limited to counting tiny things like atoms, molecules, "formula units", electrons, or photons.
• Its value can be known only to the precision that the number of atoms in a measurable weight of a substance can be estimated. Because large numbers of atoms cannot be counted directly, a variety of ingenious indirect measurements have been made involving such things as brownian motion and X-ray scattering.
Several related terms are used to express the mass of one mole of a substance.
- Molecular weight This is analogous to atomic weight: it is the relative weight of one formula unit of the compound, based on the carbon-12 scale. The molecular weight is found by adding atomic weights of all the atoms present in the formula unit. Molecular weights, like atomic weights, are dimensionless}; i.e., they have no units.
- Formula weight The same thing as molecular weight. This term is sometimes used in connection with ionic solids and other substances in which discrete molecules do not exist.
- Molar mass The mass (in grams, kilograms, or any other unit) of one mole of particles or formula units. When expressed in grams, the molar mass is numerically the same as the molecular weight, but it must be accompanied by the mass unit.
What is the formula weight of copper(II) chloride, CuCl2?
Answer: the atomic weights of Cu and Cl are, respectively 63.55 and 35.45;
63.55 + 2(25.35) = 134.45.
Answer: the atomic weights of Cu and Cl are, respectively 63.55 and 35.45;
63.55 + 2(25.35) = 134.45.
What is the molar mass of copper(II) chloride, CuCl2?
Answer: the masses of Cu and Cl are, respectively, 63.55 g and 35.45 g;
(63.55 g) + 2(25.35 g) = 134.45 g.
Answer: the masses of Cu and Cl are, respectively, 63.55 g and 35.45 g;
(63.55 g) + 2(25.35 g) = 134.45 g.
Using graph to find density of water
The density of a substance is defined as the mass divided by the volume: d=m / v. Using graphing techniques, a plot of mass vs. volume will yield a slope (Δy/Δx) of density.
Density is a physical property of a substance that does not depend on the amount of material present and is therefore called an intensive property. In this experiment, you will find the mass of water for five different volumes and plot them. Using a line of best fit, the slope will give you the value of density for water.
Always, water with different temperent has different density.
The density of water is approximately one gram per cubic centimeter. More precisely, it is dependent on its temperature, but the relation is not linear and is not even monotonic (see right-hand table). When cooled from room temperature liquid water becomes increasingly dense, just like other substances. But at approximately 4 °C, pure water reaches its maximum density. As it is cooled further, it expands to become less dense. When the water molecule makes a physical phase change its molecules arrange themselves in distinctly different patterns (Figure 8a-2). The molecular arrangement taken by ice (the solid form of the water molecule) leads to an increase in volume and a decrease in density.
Determining Aluminum Foil Thickness Lab
1. Volume of a rectangular solid V= Length × Width × Height
2. Density of a substance D= Mass / Volume
Steps
Calculate the volume of your aluminum piece.
Use the density formula, D = mass/volume for this. Solving for V gives
V = mass / density
(Volume of foil = ________ cubic centimeters = ______ ml)
Note: 1 cubic centimeter occupies the same volume as 1 milliliter.
Round your answer to match the origincal measurement that had the fewest significant digits.
(Volume after rounding for significant digits = _________________ ml)
Calculate the thickness of your aluminum.
Use the formula of a box, V = length x width x height
solve the formula for height, which represents the thickness of the aluminum foil in cm.
(h = _______________ cm thick)
Round your answer to match the original measurement with the fewest significant digits.
(Thickness after rounding significant digits = __________________ cm.)
Use the density formula, D = mass/volume for this. Solving for V gives
V = mass / density
(Volume of foil = ________ cubic centimeters = ______ ml)
Note: 1 cubic centimeter occupies the same volume as 1 milliliter.
Round your answer to match the origincal measurement that had the fewest significant digits.
(Volume after rounding for significant digits = _________________ ml)
Calculate the thickness of your aluminum.
Use the formula of a box, V = length x width x height
solve the formula for height, which represents the thickness of the aluminum foil in cm.
(h = _______________ cm thick)
Round your answer to match the original measurement with the fewest significant digits.
(Thickness after rounding significant digits = __________________ cm.)
Read more: http://www.brighthub.com/education/k-12/articles/8105.aspx#ixzz14NtZfEfy
Conclusion:
Two formulas provided above are important for the calculations. We should use them correctly to get an more accurate thickness.
Here is an example of the measurement of thin thickness thing:
http://ip.com/patapp/CN101349557
DENSITY
Density= Mass/Volume
Volume=Mass/Density
Mass= (Density)(Volume)
As you can see from the picture on the left, the rock's density caused the volume of the water to rise. The denser the solid dropped into the liquid, the higher the volume of the liquid will be.
A material will sink if its density is greater than the density of the liquid.
A material will float if its density is less than the density of the liquid.
Try these out for further practice:
1)A block of beeswax has a volume of 200.0 mL and a density of 961 g/L. What is the mass of the block?
2)A 70.0 g of manganese (density = 7.20 x 10^3 g/L) is dropped into a graduated cylinder containing 54.0 mL of water. What will be the water level indicated after the sphere is inserted?
*Remember to start Lab 2E for next day!*
Measurement and Uncertainty
Accuracy & Precision
Accuracy is the degree of veracity while precision is the degree of reproducibility.
Measurement Uncertainty
Masurement uncertainty is a non-negative parameter characterizing the dispersion of the values attributed to a measured quantity. The uncertainty has a probabilistic basis and reflects incomplete knowledge of the quantity. All measurements are subject to uncertainty and a measured value is only complete if it is accompanied by a statement of the associated uncertainty.
Absolute Uncertainty
This is the simple uncertainty in the value itself as we have discussed it up to now. It is the term used when we need to distinguish this uncertainty from relative or percent uncertainties. If there is no chance of confusion we may still simply say "uncertainty" when refering to the absolute uncertainty. Absolute uncertainty has the same units as the value. Thus it is:
3.8 cm 0.1 cm.
0.1 cm. Relative uncertainty
This is the simple ratio of uncertainty to the value reported. As a ratio of similar quantities, the relative uncertainty has no units. In fact there is no special symbol or notation for the relative uncertainty, so you must make it quite claer when you are reporting relative uncertainty.
2.95 kg  0.043 (relative uncertainty)
0.043 (relative uncertainty)
Accuracy is the degree of veracity while precision is the degree of reproducibility.
Measurement Uncertainty
Masurement uncertainty is a non-negative parameter characterizing the dispersion of the values attributed to a measured quantity. The uncertainty has a probabilistic basis and reflects incomplete knowledge of the quantity. All measurements are subject to uncertainty and a measured value is only complete if it is accompanied by a statement of the associated uncertainty.
Absolute Uncertainty
This is the simple uncertainty in the value itself as we have discussed it up to now. It is the term used when we need to distinguish this uncertainty from relative or percent uncertainties. If there is no chance of confusion we may still simply say "uncertainty" when refering to the absolute uncertainty. Absolute uncertainty has the same units as the value. Thus it is:
This is the simple ratio of uncertainty to the value reported. As a ratio of similar quantities, the relative uncertainty has no units. In fact there is no special symbol or notation for the relative uncertainty, so you must make it quite claer when you are reporting relative uncertainty.
Significant Figures
When are Digits Significant?
Non-zero digits are always significant. Thus, 22 has two significant digits, and 22.3 has three significant digits.
There are four rules for numbers with zeros:
|
Significant Digits in Multiplication, Division
 In a calculation involving multiplication, division, etc., the number of significant digits in an answer should equal the least number of significant digits in any one of the numbers being multiplied, divided etc.
In a calculation involving multiplication, division, etc., the number of significant digits in an answer should equal the least number of significant digits in any one of the numbers being multiplied, divided etc. Note that whole numbers have essentially an unlimited number of significant digits.
Significant Digits in Addition and Subtraction
Whenever we multiply or divide numbers with a finite number of significant figures the answer cannot have more significant figures than any of the original numbers. If our mouse is expected to gain 21% more weight in a month our calculator says 75.3g x .21= 15.813g is the weight gain. Since .21 only has two significant digits we must report an expected weight gain of 16 g by rounding to two significant figures.
Rounding Rules
Rule # 1:If the digit to be dropped is greater than 5, then add "1" to the last digit to be retained and drop all digits farther to the right.
For example:
3.677 is rounded off to 3.68 if we need three significant figures in measurement.
3.677 is rounded off to 3.7 if we need two significant figures in measurement.
Rule # 2:If the digit to be dropped is less than 5, then simply drop it without adding any number to the last digit.
For example:
6.632 is rounded off to 6.63 if we need three significant figures in measurement.
6.632 is rounded off to 6.6 if we need two significant figures in measurement.
Rule # 3:If the digit to be dropped is exactly 5 then:
(A) If the digit to be retained is even, then just drop the "5".
For example:
6.65 is rounded off to 6.6 if we need two significant figures in measurement.
3.4665 is rounded off to 6.466 if we need four significant figures in measurement.
(B) If the digit to be retained is odd, then add "1" to it.
For example:
6.35 is rounded off to 6.4 if we need two significant figures in measurement.
3.4675 is rounded off to 6.468 if we need four significant figures in measurement.
Remember: Zero is an even number
3.05 is rounded off to 3.0 if we need two significant figures in measurement.
Here is a video about significant figures found on YouTuBe:
Lab Day!
On Tuesday, Otober19, we did an experiment involving 3 food colourings, strips of paper, thin paper chromatography, and patience!
First we cut up a 66cm strip of paper into 3 strips of 22cm and cut one end of each strip into a point. Next we spotted the strips with a dot of food colouring each. One had a yellow spot, one had a green spot and the last one had an unknown coloured spot. Then we placed the strips (with the points facing downwards) into three test tubes containing water about 2cm high. This is where the patience comes in. We waited as the capillary action took place. It took a while but we observed that each spot started to dissolve. But after about 20 minutes, the dots had actually travelled up the strip as the water travelled up the strip also. For the yellow dot, there was no colour change. But the green dot eventually changed into yellow and blue (its component colours) and the unknown dot changed to red, yellow and blue. We then took out each strip and recorded the d1 which is the distance travelled by the solute or food colouring. We also recorded the d2 which is the solvent front or the distance travelled by the water. The class results were also recorded and we then calculated the Rf values using this equation:
Rf = d1/d2
The following picture is what our chromatography strip essentially looked like:
Click here for more detail on paper chromatography:
http://en.wikipedia.org/wiki/Paper_chromatographyHere is another way you could perform this experiment:
http://www.youtube.com/watch?v=fLc36wxLrVI&feature=relatedOn Thursday, October 21, we had our Chapter 1 and 2 test!
Separation
Hand Separation and Evaporation (Solid and Solid)
--chemanical mixture or heterogeneous mixture can be separate by using a magnet or sieve
--evaporation (solid dissolved in liquid solution)

--liquid with lower boiling pront first-vapour ascents to distillation flesk and enters condenser and then gas cools and condenses back to liquid drop the distillate as a parified liquid
Chromatography
--different materials with different speed
--mpbile phase sweeps the sample over a stationary phase
--can separate very complex mixtures
--separated components be collected individually
Sheet Chromatography
--Paper chromatography
--chemanical mixture or heterogeneous mixture can be separate by using a magnet or sieve
--evaporation (solid dissolved in liquid solution)
--boil away the liquid and the solid remains
Filtration (Solid which is not dissolved in water)
--if pores are smaller than particles, solid particles stay on filter and liquid/gaseous components pass through often used after separation by precipitation
--use filter paper (residue left in filter paper)
Crystallization (Solid in Liquid)
--a solute to solid form by cfhemical or physical change
--solids are separate by filtretion or floatation
--evaporate or cool: solid comes out as pure crystals
Gravity Separation (Solids based on density)
--a centrifuge whirls the test tube around at high speeds forring the denser materials to the bottom.
--best for small volumes
Solvent extraction (Solid and Solid)

--use liquid to dissolve one solid bur not the other so the desired solid is left behind or be solved
--solvent is insoluble with solvet already present
Distillation (Liquid and Liquid)
--heating a mixture cause low-boiling components volatilize--liquid with lower boiling pront first-vapour ascents to distillation flesk and enters condenser and then gas cools and condenses back to liquid drop the distillate as a parified liquid
Chromatography
--mpbile phase sweeps the sample over a stationary phase
--can separate very complex mixtures
--separated components be collected individually
Sheet Chromatography
--Paper chromatography
--TLC (Thin Layer Chromatography)
Separation Technique
Pure Substances and Mixtures
When we do an experiment, we often end up with a mixture of substances rather than just one. We must know how to separate the mixtures.
A single substance that has no other substances mixing with it is called a PURE SUBSTANCE. If there is something else mixed with it, it is a mixture.
Solid/liquid mixtures
The mixture of dissolved salts in water is an example of a solid/liquid mixture. The mixture is clear and no salt can be seen. We called this sort of mixture a solution. The solid which dissolves, such as salt, is called the solute. The liquid that solute dissolves, such as water, is called the solvent. Solids such as sugar and salt that dissolve are describe as soluble. Solids such as mud and coin which do not dissolve are described as insoluble.
Solute + Solvent = Solution
We often say that a solution that contains a little solute in a given amount of solvent is dilute. We called a solution contains a lot of solute in a given amount of solvent CONCENTRATED.
We called a solution SATURATED when it can dissolved all the solute. Usually cold solvents dissolve less solute than hot solvents.
A saturated solution is a solution which has dissolved all the solute, at a given temperature.
An aqueous is to describe water when it is used as a solvent. Water is the most common solvent, however the are many other solvents which we used in industry and around houses. They are all needed to dissolve substances that cannot be dissolve in water.
Liquid/liquid mixtures
We used to word MISCIBLE when liquids mix together completely in order to form one single liquid. We called those liquids which cannot mix together completely IMMISCIBLE. Oil and water form two separate layers when they are mixed.
The reason which we need to pure substance is because in Chemistry, pure substances are needed to produce drugs or perform experiments in the laboratory. However, most substances obtained from nature are mixtures. Therefore these mixtures need to be separated and purified before we can use them.
Methods of Purification and Separation
Purification and separation of substances are very important techniques in Chemistry. Some of the common methods of purification and separation are explained in the other sections.
Filtration
This is a method which is the most especially effective for separating suspensions, for example mud in water. We pour the mixture into a funnel fitted with a piece of filter paper. There are tiny holes in the filter paper for the liquid to pass through, the solid particles are too large to do so, therefore the solid particles will stay on the paper as what we called a solid residue. We called the liquid which pass through the FILTRATE.
There are two ways of folding the filter paper for the filtration:
- Fold the paper in half along one diameter then in quarters.
- Fold a fluted filter paper. Fold the paper in half, then open out, after that fold in the same director at a right angles to the original. Fold the paper two more times, the folds being all the same direction and mutually at around 45 degrees. Each section will then individually folded in the opposite direction. As result is a 'FLUTED' which sixteen faces will be produced. It provide a faster rate of filtration.
Crystallization
It is a process of forming crystals. It is also a method for separating dissolved solids from a solution.
Two common techniques of Crystallization are:
- By cooling down a hot concentrated solution.
- Slow evaporation of solution at room temperature.
When a solution of solid in liquid is heated, the liquid will evaporates. The hot vapor that formed can de condensed back to liquid again on a cold surface. We called this method DISTILLATION. Distillation is used for separating a solvent from a solution. We called the liquid collected a distillate.
Evaporation + Condensation = DISTILLATION
The water supply enters the condenser at the lower opening, leaving the upper opening to get a better cooling effect.
Chromatography
In paper chromatography, there are two factors which the movement of each substance in the mixture need to depends on.
- The solubility of the substance in the solvent. The substance moves with the solvent easily if the substance is very soluble in the solvent.
- The adsorption of the substance on the filter paper. Some solids are able to attract other substance strongly and hold them on their surface. This is called ADSORPTION. The substance will not move with the solvent easily if the substance in the mixture is absorbed strongly by the filter paper.
Chromatography can also used to identify different dyes used in food.
Floatation
An improved flotation separation apparatus for separating and classifying diverse, liquid-suspended solids having a plurality of high volume air bubble infusers. A plurality of stationary impinging plates projecting from the interior circumferential wall into the circular cavity and equally spaced circumferentially in series therealong. An injecting stream of water and air impinges upon the impinging plates in series to repeatedly create, divide and subdivide air bubbles as the injection stream transverses the series of impinging plates.
Extraction
Liquid-liquid extraction is a powerful separation technique that falls right behind distillation in the hierarchy of separation methods.
Reasons to use extraction:
Separation not feasible by distillation
Break azeotropes
Energy requirements of distillation are prohibitive
A complex distillation sequence is required
The material is heat sensitive
The material is non-volatile
Here are two videos about separation:
NAMING ACIDS
Acids are formed when a compound, composed of Hydrogen ions and a negatively charged ion, is dissolved in water. Otherwise known as aqueous (aq).
Naming Simple Acids
*simple acids are the ones that end in -ide*
1) Start with "hydro".
2) Drop the last syllable of the non-metal and replace it with "-ic".
Some examples:
HBr(aq) --> Hydrobromic acid
HI(aq) --> Hydroiodic acid
HCN(aq) --> Hydrocyanic acid
H2S(aq) --> Hydrosulphic acid
Naming Complex Acids
1) Replace -ate with -ic, and -ite with -ous.
2) End with ¨acid¨.
You can use the following sentence to help you remember the endings of complex acids:
"We ate something icky and got appendic ite ous."
Some examples:
1) HCH3COO --> acetic acid
2) HNO2 --> nitrous acid
3) H2CO3 --> carbonic acid
4) H2C2O4 --> oxalic acid
5) H2Cr2O7 --> dichromic acid
6) H2SO3 --> sulphurous acid
Here is a video for acid naming:
Writing + Naming Ionic and Covalent Compound
Periodic Table
The periodic table is divided into two major divisions: metals and non-metals. Metals (shown below in yellow) are located on the lower left and include typical metals such as iron (Fe) and nickel (Ni). Non-metals (shown in blue) are located in the upper right and include oxygen and nitrogen (common gases) as well as iodine (a solid). The position of elements on the periodic table can to used to predict with a high degree of accuracy the structure of a wide range of compounds.Types of Chemical Compounds
ionic compound is a chemical compound in which ions are held together in a lattice structure
--composed of two more particles compositely changed
--held together by electrostatic forces
--electrons are transferred from a metal to non metal
Covalent compounds between non-metals consists of 2 electrons shared between 2 atoms
--share electrons
--non metal with non metal
--use Greek prefixes to indicate the number of atoms
Rules for predicting ionic formulas
For simple binary compounds, it is possible to predict the formula of many ionic compounds by following the rules listed here.
- Is the compound ionic?
If not, none of these rules will apply. - Determine the charge of each element when present in an ionic compound.
Use the table above to determine these charges. For example, O = -2, Rb = +1. - Use the appropriate number of each ions such that:
- The sum of all charges adds up to zero.
- The simplest ratio of ions is used.
For example, if magnesium (Mg) and bromine (Br) are mixed:
- Metal (Mg) + Non-metal (Br) IS an ionic compound.
- Mg ⇒ Mg+2 and Br ⇒ Br-1
- For the final steps:
- One +2 ion is exactly balanced by two -1 ions.
- 2:1 is the simplest possible ratio.
Thus, the formula of the ionic compound formed is MgBr2.
Classes of Binary Compounds
A binary compound is one that is formed from two types of elements. Three possibilities exist.
| metal + metal | --→ | metallic compound |
| metal + non-metal | --→ | ionic compound |
| non-metal + non-metal | --→ | covalent compound |
Heating & Cooling Curves of a Pure Substance
Heating & Cooling Curves of a Pure Substance
(Temperature Changes during Phase Changes)
(Temperature Changes during Phase Changes)
Overview:
Here is a sample data of another similar lab:
Lab Notes:
A substance should be selected that will has a freezing/melting point that is well under the boiling point of water. Possible candidates - lauric acid (m.p. 44°C), acetamide (m.p. approx. 80°C), p-dichlorobenze (mothballs; m.p. 53°C).
This lab is relatively easy to set up and carry out, and may be completed in a one-hour lab period. Minimal lab equipment is required. The actual lab set-up varies with the reference used but are all similar and can be easily modified to suite the lab situation. Either the heating or cooling data may be recorded first - to record the melting phase first, either a crystallized form of the solid should be used, or the thermometer should already by "frozen" into the sample.
Questions are included in the student version, but a formal lab report may be prepared instead. An evaluation rubric for marking formal reports can be found by following the "Evaluation" link at the top of this page.
Here is a video found on YouTuBe:
After a review of atoms, ions, molecules, and states of matter, this module presents a discussion of changes of state, phase diagrams, and heating curves. The module also addresses mixtures, with a focus on separation of mixtures.
Atoms, Elements, Compounds! P. 36-39
Summary for pages 36-39:
Matter is Made of Atoms
What we know about matter is based on macroscopic observations.
Scientific explanations are not always accurate. They often provide a way to think about why things happen.
A microscopic model will help explain more about the behaviour of matter.
2-7 Atoms
Matter is composed of atoms.
An atom is the "smallest possible piece" of something.
Spheres are used by chemists to represent atoms.
2-8 Elements
An element is a substance that cannot be broken down and can exist as a solid, liquid, and gas.
Solids hold their shape because the atoms are stuck together but still vibrate.
When the temperature rises, atoms flow past each other and the liquid takes the shape of container.
At the boiling point, atoms move far apart. The liquid turns into a gas.
Particles made of more than one atom are called molecules.
Elements have different melting and boiling points. Generally, larger particles have a higher boiling point.
2- 9 Compounds
All compounds are composed of two or more more kinds of atoms.
Molecules have definite shape and composition.
A compound can be decomposed when heating and electrolysis occurs.
Not all compounds are composed of molecules.
Ions are particles with an electrical charge.
Ionis compounds form ions; molecular ions form molecules.
 |
Subscribe to:
Posts (Atom)