Matter
1.What You Know about Matter
A litre of one sample will not have the same mass as a litre of the other, because the samples have different
densities.
Properties such as color and taste describe matter specifically and can be used to identify matter.
The temperature at which matter changes from a liquid to a gas, its
boiling point, is still another property that can be used to identify matter and distinguish one kind from another.
2.Purifying Matter
mixture---two or more kinds of matter that have separate identities. Matter that is easily separated into component parts is called a mixture or said to be impure.
Tricky: Deciding which samples can be separted and which samples cannot.
Mixtures that look uniform throughour and do not scatter light are called
solutions.
Solutions like salt water and sugar water can be separated into their component parts using a procedure called
distillation.
Much of the recent awareness of air pollution, water pollution, carcinogens in foods, and similar environmental concerns have come about because new and better techniques have been found to detect those impurities.
3.Characteristics of Pure Substances
Mixtures never have a constant boiling point, but a few do.
Similar differences between mixtures and pure substances are observed when they freeze.
The temperature at which a liquid changes to a solid is called its freezing point. The freezing point is the same as the melting point, the temperature at which a solid becomes a liquid.
As time passes, the temperature of the water drops as heat is radiated to the air, and the temperature of the paradichlorobenzene drops as it loses heat to the water. The paradichlorobenzene continues to lose heat to the water when it freezes, but the temperature does not drop any further until all of it has changed from a liquid to a solid. When a mixture of paradichlorobenzene and naphthalene is cooled in water. However, when the mixture freezes, the temperature continues to fall.
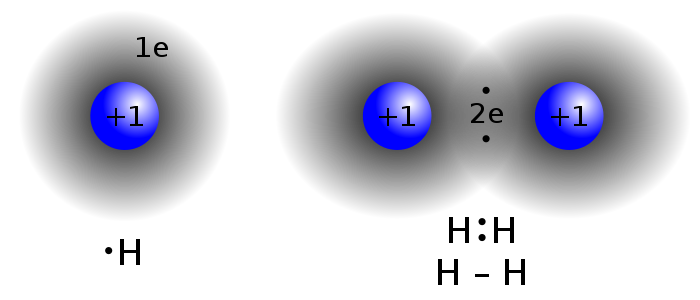
4.Chemical and Physical Changes
Changes that produce a new kind of matter with different properties are called
chemical changes. This particlar chemical change is called a
decomposition because one kind of matter comes apart to form two or more kinds of matter.
Changes that are easily reversed to get the original material back again are described as physical changes. They do not appear to produce new kinds of matter.
5.Compounds and Elements
Electrolysis involves passing an electric current through a substance, causing it to decompose into new kinds of matter.
Decomposition of a pure substance and distillation of a mixture are both processes in which matter is separated into componenes, but in thinking about what happens you see that they are fundamentally different processes. In decomposition, a single, pure substance with constant characteristic properties is somehow changed into new substances with different properties. Decomposition represents a chemical change. In distillation, the separated components exist in the original mixture as separate substances. The properties of the muixture are a blend of the properties of the mixture. Distilltion is a physical change that separates two or more things that already exist.
6.Compounds Have a Definite Composition
Not all combinations of elements are compounds.
Law of definite composition and law of multiple proportions