Periodic Table
The periodic table is divided into two major divisions: metals and non-metals. Metals (shown below in yellow) are located on the lower left and include typical metals such as iron (Fe) and nickel (Ni). Non-metals (shown in blue) are located in the upper right and include oxygen and nitrogen (common gases) as well as iodine (a solid). The position of elements on the periodic table can to used to predict with a high degree of accuracy the structure of a wide range of compounds.Types of Chemical Compounds
ionic compound is a chemical compound in which ions are held together in a lattice structure
--composed of two more particles compositely changed
--held together by electrostatic forces
--electrons are transferred from a metal to non metal
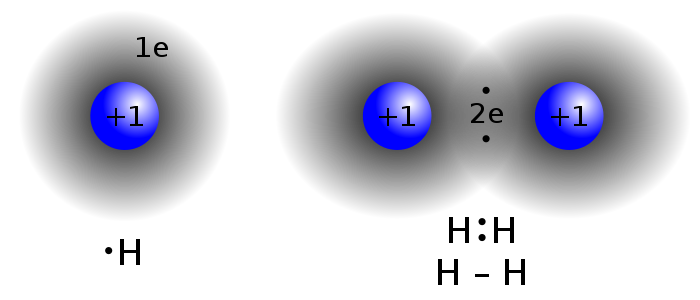
Covalent compounds between non-metals consists of 2 electrons shared between 2 atoms
--share electrons
--non metal with non metal
--use Greek prefixes to indicate the number of atoms
Rules for predicting ionic formulas
For simple binary compounds, it is possible to predict the formula of many ionic compounds by following the rules listed here.
- Is the compound ionic?
If not, none of these rules will apply. - Determine the charge of each element when present in an ionic compound.
Use the table above to determine these charges. For example, O = -2, Rb = +1. - Use the appropriate number of each ions such that:
- The sum of all charges adds up to zero.
- The simplest ratio of ions is used.
For example, if magnesium (Mg) and bromine (Br) are mixed:
- Metal (Mg) + Non-metal (Br) IS an ionic compound.
- Mg ⇒ Mg+2 and Br ⇒ Br-1
- For the final steps:
- One +2 ion is exactly balanced by two -1 ions.
- 2:1 is the simplest possible ratio.
Thus, the formula of the ionic compound formed is MgBr2.
Classes of Binary Compounds
A binary compound is one that is formed from two types of elements. Three possibilities exist.
| metal + metal | --→ | metallic compound |
| metal + non-metal | --→ | ionic compound |
| non-metal + non-metal | --→ | covalent compound |