The functional groups we focused especially on in class were: Alcohols, Halides (aka Halogens) and Nitro, Aldehydes, and finally Ketones.
Alcohols
-contain the -OH functional group
-are organic and form on saturated carbons
-are found in alcoholic drinks
To name alcohols, we simply replace the ending of the parent hydrocarbon with "ol". We also use the longest Carbon chain including the OH functional group.
Note: Always look for the name with the lowest number for the alcohol.
Examples:

Some properties of alcohols:
-parts of it are soluble, parts of it are insoluble
- smaller alcohols are soluble in water
-poisonous to some degree
If there is more than one OH group, then add the endings -diol, triol, etc to the parent hydrocarbon.
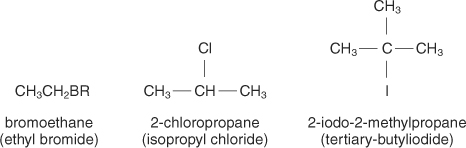
Halides and Nitro
These are attached to alkanes, alkynes and alkenes.
The most common ones are:
F= fluoro
Cl= chloro
Br= bromo
I= iodo
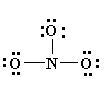
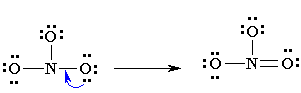
NO2= nitro
Here are some examples:
|
To name halides, we use the same rules as alkanes, alkenes and alkynes except at the beginning, we put the position and number of which halide or nitro present in the molecule as shown above. If there is more than 1 of the same type of halide, we use the prefixes -di, -tri, -tetra, etc.
Properties of Halides:
-usually insoluble in water
Properties of Nitro Compounds:
-insoluble in water
-unreactive, unless under drastic conditions
-are pretty explosive, but at least they smell good!
AldehydesThis funtional group includes a double conded oxygen on one side and an alkyl group on the other.
The oxygen must be on at the end of the chain, or else it is called a Ketone. Aldehydes are generally very reactive and somewhat soluble in water.
The ending of aldehydes is -al (from aldehyde)
Examples:
|
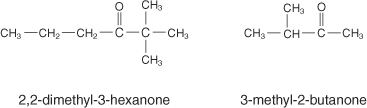
Ketones
These are similar to aldehydes, except the oxygen is double-bonded somewhere in the middle of the molecule.
The ending for this functional group is -one. To name this group, find the lowest number of the oxygen, just like the aldehydes. Ketones are usually unreactive.
Here are some examples:
 |
|
**Start studying for the Chem 11 final next week! **



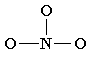
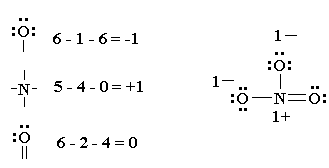





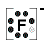
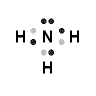 OR
OR