There are 2 types of chemical bondings:
1. Covalent bonding: a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms, or between atoms and other covalent bonds.
2. Ionic bonding: a kind of chemical bonding formed by electrostatic attraction resulting when one atom gives valence electrons to another atom, resulting in filled energy shells for both atoms.
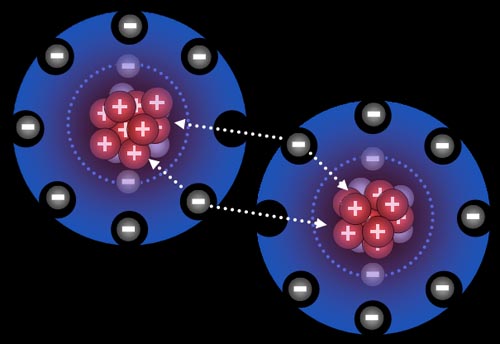
These two electrons are shared by two atoms to form covalent compound.
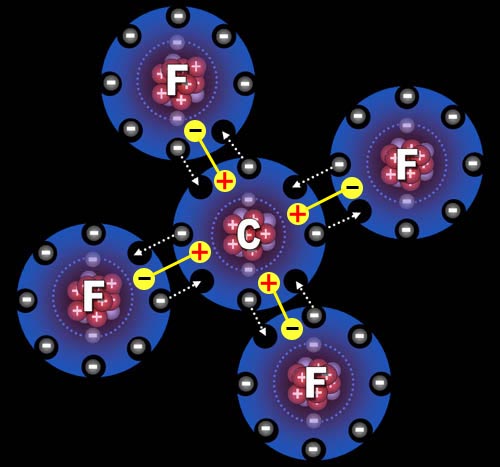
Fluorine needs one more electron to fill up its outer shell of electrons. Carbon will provide that electron as long as fluorine shares one of its electrons with carbon because carbon also wants to have eight outer electrons.
You can see how four fluorine atoms can connect to one carbon atom. Carbon is partially positive because its outer electrons are closer to the fluorine atoms and not canceling out its own positive charge. For this reason, they call this a polar covalent bond. "Covalent" because the electrons are shared and "polar" because there's a + and - charge separation.
Convalent compounds can be classified to in polar bonding and nonpolar bonding.
1. Polar bonding: Covalent compounds are said to be polar when the shared electron moves towards the atom with the greater mass.
- The atom towards which the electron pair shift gets a slight negative charge while the other atoms acquires s slight positive charge.
- The polar covalent molecules has two conters of charge and this is known as "dipole"
- There is a great difference is the electro negativity values of the atoms.
2. Non-polar bonding: Covalent compounds are said to be nonpolar when the shared pair of electrons are at an euqal distance from both the atoms invovled in bonding.
- There is no charge separation and the molecule is electrically neutral
- The eletronegativity difference between the atoms is 0.
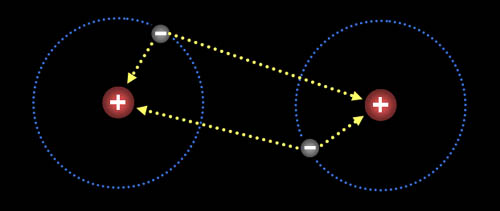 Hydrogen atoms are attracted to each other. There's a balance of repulsion and attraction. With no attraction, atoms would never make a bond. With no repulsion, the atoms would just go together as one atom. So balancing attraction and repulsion allow for atoms to form compounds.
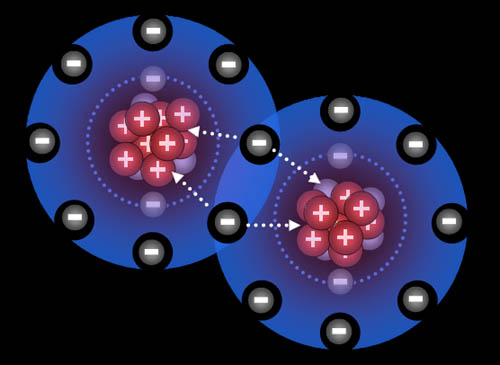
Hydrogen atoms are attracted to each other. There's a balance of repulsion and attraction. With no attraction, atoms would never make a bond. With no repulsion, the atoms would just go together as one atom. So balancing attraction and repulsion allow for atoms to form compounds.They are two fluorine atoms. The nucleus of the fluorine atom has nine protons. Two electrons orbit close to the nucleus. The outer seven spread out to maximize space between them. However there is still space for one more electron. See how the two electrons are being attracted by protons from both atoms. This pulls the atoms together, and because there is room, the atoms bond.
Here is a video found on YouTube about chemical bonding
Here is a good website to learn chemical bonding:
http://www.visionlearning.com/library/module_viewer.php?mid=55