Prelab questions:
Identify the following as physical or chemical change
a. burning wood (Chemical Change)
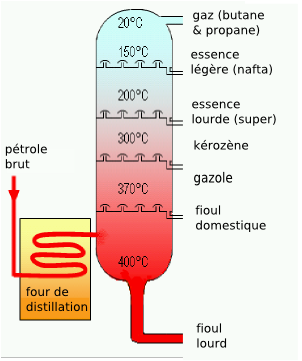
b. distillation of oil (Physical Change)
c. formation of ice (physical)
d. leaves changing color (chemical)
Properties that indicate a chemical change:
Colour, odour, solubility, phase
A similar lab found on YouTube:
Matter
What is matter?
Matter, the stuff from which our physical world is formed, presents to us as various types of material. On a first analysis, the possible phases are:
-gaseous, such as air
-liquid, such as water
-solid, such as rock
However, for classification purposes it is useful to divide materials into:
State of Matter
Matter, the stuff from which our physical world is formed, presents to us as various types of material. On a first analysis, the possible phases are:
-gaseous, such as air
-liquid, such as water
-solid, such as rock
However, for classification purposes it is useful to divide materials into:
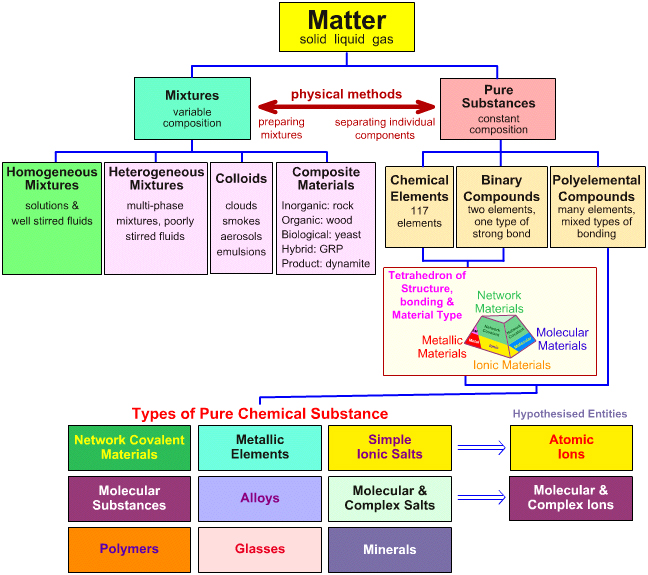
Here is the chart of matter:
- mixtures: variable composition
- pure substances: stoichiometric composition

There are 2 kinds of changes
1. Physical change
2. Chemical change
What are the differences between these two?
A physical change is a change in which no new substance is formed, for example, freezing water into ice just results in water molecules which are 'stuck' together - it's still H2O;
 |
| ice melting |
A chemical change results in the formation of one or more new substances, for example,burning wood results in ash, carbon dioxide, etc, all new substances which weren't there when you started.
 |
| wood burning |
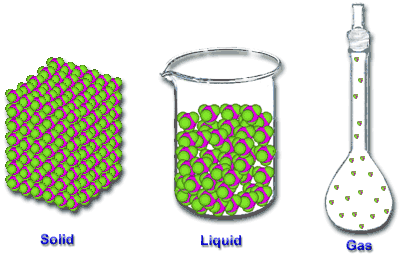
Solid - the particles (ions, atoms or molecules) are packed closely together. The forces between particles are strong enough so that the particles cannot move freely but can only vibrate. As a result, a solid has a stable, definite shape, and a definite volume. Solids can only change their shape by force, as when broken or cut.
Liquid - it is a nearly incompressible fluid which is able to conform to the shape of its container but retains a (nearly) constant volume independent of pressure. The volume is definite if the temperature and pressure are constant.
Gas - it is a compressible fluid. Not only will a gas conform to the shape of its container but it will also expand to fill the container.
Unit Conversions Review
A test on Unit Conversions is scheduled on Monday September 27. The following are some problems that are quite difficult.
Review Problems:
1. A crazy Klingon warrior eats 50 strips of dog meat for breakfast. Each dog can produce 34 strips of meat. Each strip weights 5g.
a. How many kilograms of meat do four crazy Klingon's eat for breakfast in one week?
4 klingon x 50 strips of dog meat = 200 strips/day
5 g x 200 strips = 1000g/day
1000g x 7 days = 7000g = 7kg
b. How many dogs get eaten by one Klingon per week?
50 strips of dog meat x 7 days = 350 strips of dog meat
350 strips of dog meat x 1 dog / 34 strips of meat = 10.29411765 (Round up) = 10.3 dogs
2. Convert 28.4 g/mL into kilograms per litre
28.4 g/1 ml x 1 kg/10^3 g x .001 ml/1 L=28.4 kg/L
3. Convert 872 cm ^3 into cubic meters.
872 cm^2 x 1 m^3/10^6cm^3=872 x 10^-6m^3=8.72 x 10^-4 m^3
4. (1234 km/1 hr) x (1 hr/3600 s) x (1 s/.001 ms) x (10^3 m/1 km) x (10^2 cm/1 m) = 34.3 cm/ms = 3.43 x 10^1 cm/ms
A good online unit conversion site: http://www.onlineconversion.com/
Unit Conversion video: http://www.youtube.com/watch?v=w0nqd_HXHPQ
(Beware of the boring monotone in the video above Dx)
Review Problems:
1. A crazy Klingon warrior eats 50 strips of dog meat for breakfast. Each dog can produce 34 strips of meat. Each strip weights 5g.
a. How many kilograms of meat do four crazy Klingon's eat for breakfast in one week?
4 klingon x 50 strips of dog meat = 200 strips/day
5 g x 200 strips = 1000g/day
1000g x 7 days = 7000g = 7kg
b. How many dogs get eaten by one Klingon per week?
50 strips of dog meat x 7 days = 350 strips of dog meat
350 strips of dog meat x 1 dog / 34 strips of meat = 10.29411765 (Round up) = 10.3 dogs
2. Convert 28.4 g/mL into kilograms per litre
28.4 g/1 ml x 1 kg/10^3 g x .001 ml/1 L=28.4 kg/L
3. Convert 872 cm ^3 into cubic meters.
872 cm^2 x 1 m^3/10^6cm^3=872 x 10^-6m^3=8.72 x 10^-4 m^3
4. (1234 km/1 hr) x (1 hr/3600 s) x (1 s/.001 ms) x (10^3 m/1 km) x (10^2 cm/1 m) = 34.3 cm/ms = 3.43 x 10^1 cm/ms
A good online unit conversion site: http://www.onlineconversion.com/
Unit Conversion video: http://www.youtube.com/watch?v=w0nqd_HXHPQ
(Beware of the boring monotone in the video above Dx)
Scientific Notation, with a pinch of Unitary Rates.
- used to describe very large or very small numbers using powers of 10.
 A few extra examples:
A few extra examples: 1. 4.5 X 10^3 = 4500
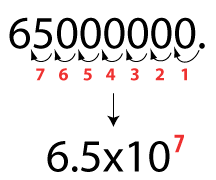
2. 6.9 X 10^6 = 6900000 3. 3.4 X 10^5= 340000
4. 8.4 X 10^9 = 8400000000 Convert from standard form to scientific notation:
Convert from standard form to scientific notation: 1. 0.000000789 = 7.89 X 10^-8
2. 3400000 = 3.4 X 10^6
 Convert from Scientific notation to standard form:
Convert from Scientific notation to standard form: 1. 6.4 X 10^8 = 640000000
2. 7.3 X 10^4 = 73000
 To convert, from Scientific notation to standard form, in a calculator:
To convert, from Scientific notation to standard form, in a calculator: 6.4 (EXP) 8
or 6.4 (EE) 8
6.4 (x10^)8
 A problem to do, with your calculator:
A problem to do, with your calculator: 1. (8 X 10^-6) - (4 X 10^-8) = 7.96 X 10^-6
2. (3.28 X 10^-5) X (5.47 X 10^9) = 1.7942 X 10^5
 A good YouTube link that shows scientific notation in detail
A good YouTube link that shows scientific notation in detailhttp://www.youtube.com/watch?v=0Dd-y_apbRw
 Unitary Rates
Unitary Rates-If 1m=100 cm1m^2=10000cm^21m^3=10 X 10^6 cm^3
-Convert: 4.3 dm^2 to Gm
dm -> m -> Gm
10^2 dm^2=1m^2
10^18 m^2=1Gm^2
Subscribe to:
Posts (Atom)